
Tech Articles
Volumetric method and gravimetric method are the two primary methods for the determination of equilibrium sorption isotherms. In the volumetric method, the hydrogen sorption or uptake is measured by monitoring the drop in hydrogen pressure in a system of a fixed, known volume, with desorption being monitored by an increase in pressure. The volumetric method is also known as the Sievert’s Method or Manometric Method. In the gravimetric method, the hydrogen uptake is measured by monitoring the weight of the sample following a step change in the hydrogen pressure. A series of isotherms measured at different temperatures can then be used to calculate certain thermodynamic properties of the hydrogen solid system.
Van ‘t Hoff plots are a commonly used method for determining the thermodynamics. It is critical for thermodynamic analysis that the measurement of a PCT isotherm is performed in such a way that the hydrogen gas, and hydrogen storage media have attained equilibrium before recording Peq or (for hydrides) the equilibrium plateau pressure. Achieving true equilibrium pressures is what separates out (and removes) any slow kinetics effects from the pressures that are used to create van ‘t Hoff plots and, ultimately, determine reaction enthalpies and entropies.
To be able to derive van ‘t Hoff plots accurately from PCT isotherms it is important that enough data points have been taken in each PCT isotherm to be able to clearly identify plateau pressures and distinguish phase transitions. In volumetric PCT measurements it is recommended that at least 30 but preferably as many as 100 steps in concentration (data points) be taken for each isotherm (with at least 5-10 data points per plateau). This is equally true of gravimetric measurements. However, the fact that gravimetric PCT measurements take steps in pressure rather than concentration means that it may be difficult to determine exact equilibrium pressures for materials with flat plateaus (complex hydrides for example). This is because a material may go from the unhydrided state to the completely hydrided state in one pressure step (as there is no limit on the amount of hydrogen to which the sample is exposed). The equilibrium pressure for a given sample temperature will be somewhere between the final pressures before and after the hydriding (or dehydriding) pressure step. In this case the accuracy of the equilibrium pressure is a function of the size of the pressure step that is taken rather than the accuracy of the measuring pressure transducer.
An important distinction between gravimetric and volumetric methods is that gravimetric instruments make PCT measurements by incremental changes in pressure, whereas volumetric instruments make PCT measurements by incremental changes in concentration. Thus, in gravimetric PCT measurements one has control over the y-axis (pressure) but essentially no control over concentration. For materials with relatively flat equilibrium plateaus (alanates, borohydride… essentially reversible chemical hydrides) an incremental increase in pressure from slightly below the plateau to just above the plateau will fully hydride the sample. The inverse is true for desorption. This is why gravimetric equilibrium PCT measurements on such materials often have no data points actually on the plateau. The plateau pressure can thus be inferred to be between the highest pressure of the sample in its unhydrided state and the lowest pressure in its hydrided state.
Volumetric Method
For routine hydrogen sorption measurements of porous materials, the volumetric method predates gravimetric method, primarily owing to the technological developments necessary for the latter, in terms of microbalance technology and the need for computer control. However, for volumetric method, it is important to accurately determine the sample skeleton volume (or skeletal density), which could cause large errors in the calculation of the heats of adsorption. In practice, He, which is assumed to have negligible adsorption on the substrate at room temperature and low pressure, is usually employed as the calibration gas and the volume difference between the measured volume of the sample cell before and after loading the sample into the cell at room temperature is the sample skeleton volume.
The following are important issues that need to be addressed in making accurate volumetric measurements in particular for physisorption materials at low temperatures and high pressures.
1. Temperature Correction
The excess H2 storage capacities in porous MOFs are often measured at cryogenic condition (77 K). The huge temperature difference between the gas reservoir and sample holder needs to be corrected based on the methods discussed above. In practice, it is necessary to soak the sample holder in liquid nitrogen bath for 30 min or longer to reach a thermal equilibrium. In addition, with simple Dewar systems, a deeper or larger flask is recommended because it can keep the temperature longer. An important consideration is that if the cryogenic liquid level in the Dewar changes significantly during a measurement, the gas temperature (density) gradient will also change, causing a change in the “apparent volume” and thus errors in the measurements. Cryogenic systems that maintain a constant cryogenic temperature zone either through LN2 refill or by cooling through Cryo-evaporation are more stable and will remove or significantly reduce this error.
When it comes to room temperature measurement, the environmental temperature fluctuation will also affect the results. It would is recommended to keep all components of the equipment (including sample holder) in a constant temperature environment.
2. Temperature Measurement
It is often the case with volumetric instruments that the thermocouple used to measure sample temperature is not in direct contact or, at least, in the middle of the sample. In many cases the thermocouple is in the furnace (or dewar, or cryostat) surrounding the sample holder. This is unlikely to give an accurate reading of the actual sample temperature because of heat leaks through the gas tubing, convection, etc. This problem becomes worse as the sample temperature is further from room temperature. Typically the measured temperature of the furnace is set using a calibration table or function to account for the difference in temperature at the point of measurement and the actual temperature of the sample. However, even a slight change in the position of the sample holder in the furnace (dewar) can cause a significant (as much as 10-20%) variation in the actual temperature of the sample. This, in turn, will cause large errors in the determination of heats of reaction by the van ‘t Hoff method. Bogdanovic et al. reported an error of ±1.7 kJ/mol H2, or 2.4%, based simply on an uncertainty of ± 0.5 oC in the sample temperature.
The take home message is that temperature measurements that are not taken from within, or at least, in close proximity to the sample are probably unreliable and certainly will not be able to register temperature changes in the sample due to exothermic or endothermic reactions.
3. Free space measurement
One of the biggest problems encountered for high-pressure sorption measurements is the determination of the dead volume. Since porous MOFs are usually microporous, the helium uptake during the free space measurement is almost inevitable. In addition, there is often a large difference between the free space determined via adsorption vs. desorption. The difference of free space obtained can easily impose 10% error on the final result. In order to get an accurate result, the free space used for temperature correction to the adsorption isotherms should be obtained through adsorption data, and more than ten separate dead volume measurements should be carried out to get an accurate mean value.
4. Sample Quantity
The effect of sample quantity on the result is not straightforward. Small amounts of sample give less of an error on the dead volume determination, but pressure changes due to sorption are subsequently smaller, leading to larger errors in the final result. Larger quantities of sample give more accurate and reliable reading on the pressure change, but would also introduce more error on the dead volume determination. Generally, dead volume measurements using Helium at room temperature provides enough accuracy that the advantage of higher accuracy in the pressure change measurement is significantly more important. Thus, larger amounts of sample are almost always preferred.
5. Sample Handling
If the porous material has high H2 storage capacity, it normally also means it will adsorb moisture in the air too. In order to prevent contamination from the air, all sample handling should be carried out in a glove box and then sealed before removal. Before measurement, samples should be degassed on the equipment at high temperature overnight to completely remove any adsorbed moisture.
6. Leak Checks
Hydrogen leaks in high pressure equipment not only affect the results, but can also be very dangerous regardless of the type of measurement equipment being used. All gas fittings and seals should be checked regularly (every measurement) to prevent gas leakage. One can easily identify a gas leakage during the free space determination. If there is huge difference between different runs, that typically means a gas leakage. In addition, a flammable gas detection system is very helpful during the H2 measurement.
7. Full Isotherm Van ‘t Hoff Measurements
The classical method for determining the thermodynamics of hydride formation and decomposition is to make a series of PCT isotherm measurements at different temperatures. Then to create a van ‘t Hoff diagram by plotting the natural log of the plateau pressures vs. 1/sample temperature. This is illustrated in below figure 1 for a series of PCT desorption measurements on LaNi5.

Figure 1: Standard method of making a Van ‘t Hoff plot from a series of desorption PCT measurements at different temperatures LaNi5
Using the volumetric measurement technique dosing takes place in increment concentration steps, unlike in the gravimetric technique where data is collected in incremental pressure steps and there may or may not be actual data points on the plateau depending on the intrinsic slope of the two-phase region (alloy and hydride). The volumetric measurement technique is advantageous for van ‘t Hoff analysis for two reasons. The first is that this method is very accurate in identifying plateau pressures at exact and consistent hydrogen concentrations. The second is that, at those concentrations the pressure time profile can be observed to ensure that the reaction has reached equilibrium. In fact, for materials with poor kinetics, it may be possible to use the pressure / time profile to extrapolate to a theoretical plateau pressure without waiting an unreasonable amount of time (more than a few days) to reach equilibrium for each gas dose.
As mentioned earlier, it is important to collect many data points along the PCT isotherm. One reason is that more than one hydride phase may be formed with very similar heats of formation. Large dosing steps, or the pressure stepping process in the case of gravimetric measurements are likely to miss these details. This is one reason why the volumetric method of having many concentration data points on a PCT plateau is advantageous. In such a case it is necessary to take very small steps in concentration and wait sufficiently long at each step to reach equilibrium. An example of this is shown in Figure 1 and detailed in Figure 2. PCT measurements (left) were made using very small steps in concentration (small volume doses and small pressure steps) on a LaNi5 sample at different temperatures. The ability to collect many data points along the isotherm made it possible to observe the formation of a second meta-stable hydride phase (which has been identified as the gamma-phase by Ono Figure 2 and others). This is important, because, had only a few (e.g. 10) data points been taken the step in the plateau pressure may not have been observed. The potential effect on the determination of the entropy and enthalpy of hydride formation are shown schematically in a simulated van ‘t Hoff plot on the right side of figure 2. The difference in the equilibrium pressures between the two plateaus (orange vs. green) is significant and if incorrectly ascribed to one hydride or another (or in the case of only a few data points; averaged between the two) would lead to inaccuracies in the determination of the entropies and enthalpies of hydride formation.

Figure 2: Illustration of detailed volumetric desorption PCT measurements at different temperatures for LaNi5 (left) and different possible van ‘t Hoff plot derived from these isotherms (right).
8. Micro-Volumetric Full Isotherm Measurements
Occasionally samples are too small (1-100 of mg depending on H2 capacity) to be measured on a standard volumetric instrument. This has been the circumstance for some thin film samples and materials that are difficult to synthesize in large quantities, as was the case in the early days of single wall carbon nanotubes. In such cases a specialized micro-volumetric instrument was used to perform PCT measurements in much the same manner as a typical full sized volumetric instrument.
Figure 3 shows a series of PCT measurements of a thin-film sample of palladium/nickel (Pd deposited on thin Ni film and removed from the substrate) using a Sievert’s-type instrument with a MicroDoser attachment. Not only was the sample relatively small (91.3 mg), but palladium-hydride has a relatively low hydrogen content (0.6 wt.%). Thus, the PCT measurements involved extremely small quantities of gas. The number of data points along the PCT curves demonstrate the ability to dose the sample with very small quantities of hydrogen. This is a necessary prerequisite for being able to perform a “Direct van ‘t Hoff” measurement on this sample.

Figure 3: A series of absorption and desorption PCT measurements at 38°C, 110°C and 200°C on a thin-film Pd/Ni sample removed from the substrate
Focusing on these full PCT measurements for the moment, one can see that there is a clear hysteresis between absorption and desorption measurements. To understand whether this was a materials effect or an artifact of non-equilibrium measurements, it is necessary to look at the pressure-time data recorded during the automatic dosing process. For this experiment, the equipment allowed efficient exponential time data collection an equilibrium test function to be performed to reduce the quantity of data collected and speed up the total isotherm collection time. The equilibrium test function required the instrument to only stay within a given dose until the approach to equilibrium test condition was met (i.e. rate of absorption or desorption falls below a selected value).
Figure 4 shows a portion of the pressure time sequence of doses collected in the beginning of the absorption PCT at 38°C. The data demonstrates that the absorption at each dose has reached a sufficiently steady pressure. This means that the sample’s hydride phase can be considered to be in equilibrium with the gas phase at the final pressure reading of each dose (equilibrium pressure). It is this final pressure reading that corresponds to the plateau pressure in the corresponding PCT plot at the measured hydrogen concentration.
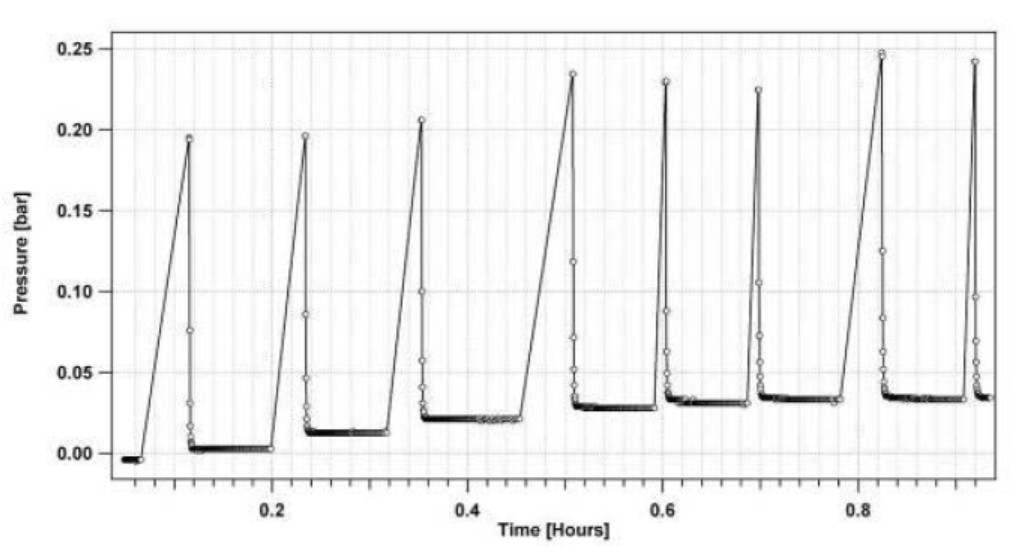
Figure 4: Portion of pressure vs. time data for a sequence of doses of hydrogen during an absorption PCT measurement at 38°C.
Figure 5 shows similar pressure vs. time data across the plateau region of the desorption PCT at 200°C. Note that data is collected very rapidly at the beginning of each dose. In some cases, the first data point of each dose is taken just as the dosing valve opens, before the gas pressures between the dosing reservoir and the sample volume have equalized. Thus, the actual reservoir pressure is observed at the beginning of many of the individual doses.

Figure 5: Pressure vs. time data for the plateau region of the desorption PCT measurement at 200°C.
The important point of these figures is to show that for every dose the instrument waited a sufficiently long time for the desorption to reach an equilibrium state. This is demonstrated more clearly by focusing in on an individual dose of Figure 5.
9. Direct Van ‘t Hoff Measurement Technique
The issue, however, is that even for materials with relatively good absorption and desorption kinetics (e.g. LaNi5 in the example), collecting a series of PCT measurements may take from several days to weeks. Even worse, materials with moderate to poor kinetics may take many weeks to months to perform a proper series of equilibrium thermodynamic PCT measurement.
Fortunately, well designed volumetric equipment can allow one to perform what we term here a “Direct van ‘t Hoff” measurement. This method is efficient in that the measurement time can be reduced to less than a single PCT measurement. The technique is only accessible to volumetric measurement systems. However, there are important caveats and precise control over the experiment is absolutely necessary. The method is explained here using hydrogen desorption from LaNi5 as an example.
With the “Direct van ‘t Hoff” method, it is important to ensure that concentration does not change significantly during the temperature stepping. As the temperature is increased the sample will desorb hydrogen until the equilibrium pressure is attained. This means that the sample’s hydrogen content will change. In-other-words, it is important to maintain a relatively constant concentration and to certainly avoid the sample falling off the plateau.
10. Physisorption Measurement Systems
Hydrogen physisorption measurements are often performed with a volumetric system involving successive gas expansions from a reference to a sample cell as shown in Figure 6. Schematic view of a volumetric system.

Figure 6: Schematic view of a volumetric system
The majority of physisorption storage measurements are carried out at low temperatures (typically 77K). Because of the extreme temperature differential between the reference cell and pressure transducers, temperature monitoring, control, and in particular, stability are very important in all temperature and pressure ranges of physisorption measurements. For low temperature measurements, one common setup is to place the sample cell inside a temperature controlled cryostat operated using liquid nitrogen Figure 7. It is critical to understand that if a basic liquid Nitrogen (or Argon…) dewar is used to keep the sample at 77K, the liquid Nitrogen level must be kept constant (either by topping up the dewar or by moving the dewar). If not the temperature gradient will change with time and will typically have a significant affect on the results that is difficult if not impractical to correct. Because of this complication, a temperature controlled cryostat is often used instead of a dewar. A cryostat will also enable measurements at temperatures other than that of the cryogenic liquid.
An advanced system of control over the static (not changing in time) gas temperature gradient can be achieved by creating a quasi-discreet temperature change in the tubing between the cryogenic temperature of the sample cell and the elevated temperature (typically room temperature) of the gas dosing volumes. This is done by fixing a second temperature control system (also shown in Figure 7) on the gas tubing just above the sample cell. A small heating element allows heating on a small length of the tubing to keep the major part of the gas at, or near, room temperature with minimal perturbation of the cell’s temperature. It also allows for a nearly discrete temperature change in the gas rather than an extended temperature gradient which is more difficult to compensate for in the gas law calculations of hydrogen mass balance. The sample cell’s controlled heating element enables the sample temperature to be controlled at temperatures above that of the cryogenic liquid (typically T> 77K). This enables adsorption and desorption isotherms to be collected at several sample temperatures which is required to determine the isosteric heats of adsorption. Clearly accurate measurements of the sample’s temperature is essential for meaningful thermodynamic analysis.

Figure 7: Schematic view of the cryostat
Measurements of excess hydrogen adsorption isotherms at temperatures ranging from 83 to 273 K based on the above system has allowed the accurate determination of isosteric heats of adsorption of several microporous materials by applying the Dubinin-Astakhov (DA) model.
Gravimetric Method
The gravimetric method is often used to determine thermodynamic information indirectly through PCT measurements. The plateau pressures from multiple isotherms can be compiled into a van ‘t Hoff plot. The van ‘t Hoff equation can be used together with a van ‘t Hoff plot to determine ΔHf and ΔSf for a reaction.
1. Hysteresis in Reversible Hydrides
However, these measurements are not always as straightforward as they may seem. As mentioned, hysteresis can lead to a different ΔH for the sorption and desorption reactions, so it is important to specify whether absorption or desorption values are being reported. While some hysteresis may be an intrinsic property of the storage materials, it can also be due to not achieving equilibrium conditions. In particular, materials with large heats of formation will produce significant heat on adsorption heating the sample and cooling on desorption. Thus, for the measurement to achieve equilibrium, the sample must return to the set temperature by transferring heat. This can be difficult to achieve where the only thermal contact the sample has is with the large volume of gas as is the case in gravimetric measurements. While hysteresis effects can be reduced by allowing a longer time to reach equilibrium at each measurement point, some materials have such poor kinetics that this is impractical.
2. Temperature Measurements
Accurate sample temperature measurement is critical to correctly determine enthalpies of reaction (and isosteric heats of adsorption) using PCT measurements. This may be a significant issue with gravimetric measurements due to the difficulty of obtaining the exact sample temperature measurements. This is because, generally speaking there is no thermocouple in direct contact with the sample in most gravimetric systems. Note that this is also often the case with volumetric instruments that do not have a thermocouple in the middle of the sample as mentioned above.
Highly exothermic materials lead to even greater difficulty, since a typical sample temperature may fluctuate considerably during hydrogen uptake and release. In this case the equilibrium pressure will correspond to the sample temperature which may be far from the applied pressure. Thus, the assumed sample temperature and measured pressure may not correspond well at all to the actual hydrogen uptake conditions actually being experienced by the sample itself. In volumetric instruments this can be remedied to some extent because the sample is generally in direct contact with the vessel walls allowing greater heat transfer. It should be noted that this factor is somewhat muted by the fact that powder samples typically have poor heat transfer properties. Possibilities to mitigate these effects through enhanced heat transfer methods of the Kinetics chapter. However, with gravimetric measurements the samples are typically suspended in the gas, excluding the possibility of significant heat transfer, except through the gas itself.
3. Buoyancy
Buoyancy corrections have such a serious impact on the accuracy of gravimetric measurements. One common error is to introduce the adsorbate density in the corrections and quoting the uptake results as an excess uptake which it is not. There is also the risk of using an arbitrarily low adsorbate density and reporting an unrealistic uptake value.
4. Other Considerations
Other important issues to be considered when making gravimetric measurements are:
- The impact of impurities in the hydrogen gas
- Foaming or material loss of the sample
- Evolved gases (e.g. solvents, water, ammonia…) from the sample during bake out or desorption measurements
- Gas leaks out of (high pressure) or into (sub-ambient) into the instrument
- Sample temperature measurements
- Adequate heat transfer to or from the sample
- Airless transfer of the sample into the instrument
- Volume dilatation of the sample on heating or hydrogen absorption
These are important considerations that should be well understood before making measurements.
5. Gravimetric Thermodynamic Measurements
One important distinction between gravimetric and volumetric measurements of PCT isotherms is that in the gravimetric method data is collected in incremental steps in pressure (slices along the pressure axis of the PCT plot) whereas in the volumetric method data is collected in incremental steps in concentration (slices along the concentration axis of the PCT plot). This has an important consequence for thermodynamics measurements. The classic van ‘t Hoff method determines enthalpies and entropy from a series of PCT isotherms. This method relies on using equilibrium pressure and temperature data at constant concentrations. The pressure and temperature data will be as accurate as the sensors that are used to measure them. However, concentration in a gravimetric measurement will be determined by the pressure step which is a function of the pressure control device, not the pressure measurement transducer. For most materials that have sloping isotherms this will not be a problem because Peq values (for a given temperature) can be adequately interpolated between data points at different (but close) concentrations. However, for hydrides with relatively flat plateaus it is generally not possible to collect data points on the isotherm. This means that the Peq will have to be estimated to be between the two endpoints of the isotherm and therefore the accuracy of the Peq value is dependent on the size of the gas dosing steps rather than on the accuracy of the pressure transducer. Obviously the thermodynamic results will be more accurate for measurement made with the smallest dosing steps the instrument can perform. However, very small pressure dosing steps may require substantially more data collection time.
Reference List:
Reif, F., “Chapter 5 – Simple applications of macroscopic thermodynamics”, Fundamentals of Statistical and Thermal Physics, McGraw-Hill.(1965), ISBN 0070518009
Zhou, W., Wu, H., Hartman, M.R., Yildirim, T., “Hydrogen and Methane Adsorption in Metal-Organic Frameworks: A High-Pressure Volumetric Study”, J. Phys. Chem
Lee, Y.-W., Clemens, B.M., Gross, K.J., “Novel Sieverts’ type volumetric measurements of hydrogen storage properties for very small sample quantities”, J. Alloys and Compounds
Bogdanovic, B., Bohmhammel, K., Christ, B., Reiser, A., Schlichte, K., Vehlen, R., Wolf, U., “Thermodynamic investigation of the magnesium-hydrogen system”, J. Alloys and Compounds
Ono, S., Normur, K., Akiba, E., Uruno, H., “Phase transformations of the LaNi5-H2 system”, J. Less-Common Metal
Collaboration with Pivak, Y., Schreuders, H., Dam, B., and Griessen, R., Vrije Universiteit Amsterdam, The Netherlands
All Rights Reserved by Gold APP Instruments Corp. Ltd.
WeChat WhatsApp


GOLD APP INSTRUMENTS CORP. LTD.
HongKong Add: Flat Rm A17, Legend Tower, No. 7 Shing Yip Street, HK, China
Mainland Add: R1302, Baoli Tianyue, Shaowen Rd., Yanta Dist., Xi'an 710077, China
T: +86-182 0108 5158
E: sales@goldapp.com.cn