
Tech Articles
This long-term performance behavior is often referred to as degradation behavior. Two general approaches have been taken with respect to the measurement of the long-term stability of hydrides. One approach (Hydriding-dehydriding) Cycling Tests evaluates the ability to maintain properties, such as hydrogen capacity operating temperature and/or pressure after numerous hydriding-dehydriding cycles. Another approach, Aging Test, has been to evaluate the hydride’s performance after is maintained at very high pressure and temperatures for a long period of time. These may be further divided into: (1) Intrinsic and (2) Extrinsic testing. All intrinsic test involve using pure hydrogen, and extrinsic tests involve gaseous impurities (generally in ppm levels to simulate nominal hydrogen gas composition) mixed with hydrogen gas. The different types of degradation testing are laid out below in Figure 1.

Figure 1: Chart of hydrogen storage materials stability testing methods
As shown above, cycling tests are comprised of: (a) Thermal Cycling (constant pressure), (b) Pressure Cycling (constant temperature) and (c) Pressure-Temperature cycling, referred to as P-T cycling in which both the pressure and temperature are varied, but the volume of hydrogen added to the samples is fixed. P-T tests are important, for example, in heat pump applications in which a certain volume of hydrogen gas is transferred from a high pressure hydride to a low pressure chamber, and back and forth. In cases where the fresh hydrogen needs to be recharged frequently, for example in hydrogen powered vehicles, (extrinsic) pressure cycling tests are used. In these tests one uses ultra high purity (UHP) hydrogen premixed with ppm levels of impurity gases that are generally be encountered in modern hydrogen refueling stations.
1. Pressure Cycling Tests
The most direct way to measure the Cycle-Life performance of hydrogen storage materials is to perform a series of full absorption and full desorption measurements with the material at one set temperature and with fixed charge and discharge pressures. For hydride systems with a plateau, the charge pressure would be set above the plateau and discharge pressure below the plateau. Such measurements can be made using either volumetric or gravimetric instruments. However, because the measurements may be performed over 10’s, 100’s or 1000’s of cycles, instrument stability over very long periods of time is essential. For this reason, volumetric (manometric) instruments are typically used. At its most basic level, this involves a sample cell of known volume, and separate calibrated dosing volumes for absorption (smaller) and desorption (larger) with a dosing valve between the dosing volume and sample cell. The amount of hydrogen uptake or release of hydrogen is determined from the change of pressure measured upon exposing the sample to high pressure (absorption: smaller calibration volume) or low pressure (desorption: larger calibration volume).
This method has the advantage that fresh hydrogen gas is being used with each cycle, which most closely represents real world applications. However, while this relatively simple type of measurement provides a first look at the cyclic stability of reversible hydrogen storage materials, it does not necessarily simulate how a material will actually be used (for example in a hydrogen powered vehicle). Real world use in a vehicle will have many partial discharge and refills and the hydrogen release will most likely vary greatly with the demand and use of the vehicle. In other words, the material will experience dormant periods, then be heated for delivery of hydrogen with varying endothermic excursions from a steady state temperature. These delivery variations will take place over relatively long periods of time. Charging, and the associated exothermic release of heat combined with cooing are expected to occur in a matter of minutes on refilling. Real world conditions will certainly be very different than initial simplified Cycle-Life experiments based on a series of isothermal full absorption/desorption cycling measurements. The cycling conditions themselves are likely to have a very strong impact on the Cycle-Life characteristics of the material.
2. Pressure-Temperature Cycling Tests
Pressure-Temperature Cycle Tests are a step closer to actual application conditions. These tests are performed in the same manner as pressure cycling measurements but change the sample temperature as well as pressure on each absorption and desorption. For absorption the charging pressure is increased and temperature decreased to conditions that would be found at a filling station. For desorption the pressure is lowered and temperature increased to onboard hydrogen delivery conditions. As with pressure cycling tests, fresh hydrogen is usually used with each cycle.
3. Thermal Cycling Tests
Pressure cycling, as described above, can be very time consuming and involves the consumption of large amounts of gas and potentially the change out of gas cylinders introducing the potential for unknown factors. For example, one assumes that all gas cylinders are of consistently high purity, but this is not guaranteed. Also, when a gas cylinder is removed and replaced with a new one, it is imperative that all lines, valves and pressure regulators that have been exposed to air in the process are evacuated if not at least thoroughly purged of air and impurities before continuing with the cycling experiments.
Pressure cycling also involves the functioning of mechanical devices (valves) with each cycle. While this is closer to real world applications, it may not necessarily be the most efficient or effective way to get preliminary performance data. For this reason, Cycle-Life performance is sometimes characterized by thermal cycling. The advantage of this method is in its simplicity and the (near) certainty that no gaseous impurities are introduced with the hydrogen for more than just the first charging.
The basic measurement apparatus involves one (known) volume at a known temperature connected to a sample cell of known volume and a pressure transducer to record pressure changes on absorption and desorption. The sample cell is then heated to release hydrogen and cooled to absorb hydrogen into and out of the calibration volume. The gas is never changed and there are not necessarily any dosing valve operated during the measurements. The sample is simply heated and cooled repetitively and the amount of hydrogen released and absorbed is measured with each cycle. Lambert et al. performed degradation tests on LaNi5 samples micro-alloyed with different elements using such an apparatus. Figure 2 A schematic of this apparatus is shown in . One (heated) vessel was filled with the sample and the other was an attached vessel to receive or supply the volume of discharge or absorbed hydrogen. This device had two different calibrated reservoir vessels (75 and 500 cc). The larger vessel was used for samples with plateau pressure near 1 bar at room temperature the smaller vessel was used for sample with much higher plateau pressures. This allowed for a reasonably measureable pressure change on going from a fully hydrided to un-hydrided state on both low and high plateau pressure samples.

Figure 2: Thermal Cycling Apparatus Heating cooling apparatus for long-term thermal cycling
Absent the effect of impurities, these tests are useful in examining the intrinsic degradation of the storage material due to hydrogen uptake and release as well as due to temperature changes and possibly induced thermal gradients.
4. Closed System vs. Fresh Gas Tests
The disadvantage of pure thermal cycling versus pressure, or pressure-temperature cycling is that in thermal cycling the same gas is always used so the effect of impurities that one finds in commercial hydrogen is not introduce in each cycle as it would be in many real applications. However, extensive closed cycling does correspond to the operation of hydrides in heat pumps and sorption cryocoolers, It also can be significantly different from actual applications in that the hydrogen pressure is the resulting equilibrium pressure rather than the applied pressures that would be found in an actual application. In the case of hydrides the pressure essentially follows the van ‘t Hoff plot, so there is not the driving potential of a large over- or under- pressure applied to the materials. This in itself, may have a considerably different impact on internal stress, defect formation, and ultimately the Cycle-Life stability of hydrogen storage materials as compared to pressure, or pressure-temperature cycling tests.
The advantage of this method is the simplicity and reliability of the measurement system. It reduces the number of conditions, potential mechanical issues, and actual operations involve. These are usually fully automated measurements that can be performed for long periods of time without much operator intervention. An important aspect is that it also quickly supplies base-line information about a material’s performance without the introduction of additional factors such as gas impurities. These measurements can then be compared with pressure, or pressure-temperature cycling results (with and without controlled impurities) to get a clearer picture of what has the strongest influence on a material’s storage properties degradation.
5. Aging Testing
The long-term stability tests using the above cycling methods are generally very lengthy. To minimize the total experiment time and get a quick idea of property changes of hydrides one can use Aging Tests. These are typically short-duration tests. While in actual applications, charge/discharge cycling ideally takes place at near ambient conditions (although, perhaps up to 200°C and 100 bar H2 pressure), in Aging Tests, experiments are generally performed at considerably higher pressures and temperatures to accelerate degradation processes.
Lambert et al. also performed thermal aging tests on substituted LaNi5 samples. About 12 grams of the intermetallic samples were ground and loaded into a sample holder. The samples were first subjected to 10 activation cycles on a standard Sievert’s apparatus and then absorption and desorption PCT isotherms were taken at 25°C. The samples were then transferred to their thermal aging apparatus and heated to 180°C and a pressure of 193 bar. The samples were held in this condition for 256 hours and the pressure increase was monitored as an indication of the decrease in H/M content of the hydride. A desorption PCT was then measured after the sample had been cooled to 25°C. A schematic diagram of their apparatus is shown in Figure 3. A metal-hydride compressor with ultrapure hydrogen source was used to supply hydrogen pressures as high as 207 bar to the sample.

Figure 3: High pressure thermal aging apparatus
For hydrides, selection of the thermodynamic parameters for these tests depends on the nature of isotherms. It is recommended that the aging be performed in fully hydrided phase, above the plateau pressure. An age-testing pressure envelope is shown in Figure 4. A pressure concentration parabola is drawn to encompass initiation and completion of the two (α+β) phase region. In these aging tests the mid-plateau H/M is determined, and the pressure and temperature are maintained near the top of the parabola for faster response. The actual experimental conditions are generally, 10-25% lower pressures than the apex of the parabola, depending on the nature of the materials to be closer to the plateau pressure.
These tests may be classified as Short-Duration tests, conducted for 1 to 3 weeks to give a reasonable idea of the hydriding behavior of the alloy/compound. By comparison, detailed cycling tests may take several months. The situation will vary from alloy to alloy, especially if the alloy results in an amorphous hydride. In some important examples of such research, Aoki and Jai-Young Lee’s group have reported detailed investigations of Laves-phase amorphous hydrides. Note that it is a good practice to check the phase diagrams, if available, of the alloys being studied to be aware of any phase transitions of the parent metal/alloys, or hydride themselves that may occur under such aging conditions.
The idea of these Short-Duration tests is to stress the material/hydrogen sorption process in a way that extrapolates to the results that would be obtained by long-term cycling. The basic purpose is to observe if any, and to what degree, hydrogen-storage properties degradation has taken place. The process of aging is shown in Figure 4 in a schematic using a pressure-composition isotherm (left) and pressure vs. time schematic (right).
An absorption isotherm is obtained (after initial activation cycles), and the sample chamber is sealed at a temperature T1(oC) with β phase hydride at H/M =1. Then the sample temperature is quickly increased to a temperature,T2, which is associated with a pressure increase (and likewise a loss of hydrogen capacity, the magnitude of which varies from alloy to alloy). After the sample achieves a temperature of T2, the aging process starts as shown on the right in Figure 4. If the hydrogen pressure increases over time, then the H/M in the sample also decreases as a function of time. The capacity loss due to this aging process is an indicator of the degradation that might be expected from hydrogen absorption / desorption cycling of the same material.
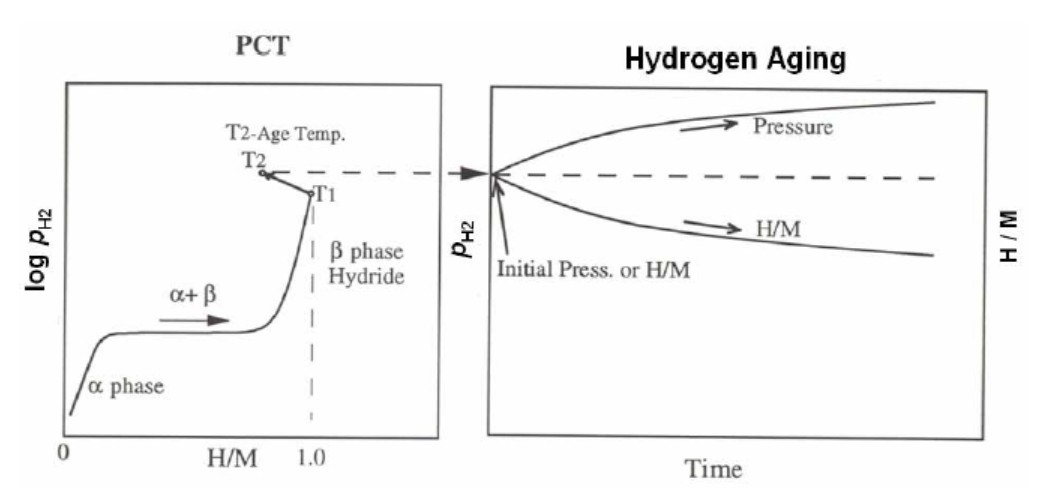
Figure 4: Thermal aging method is shown in the schematic. (Left) An isotherm is generated at a temperature T1 until fully saturated in the β phase (above the plateau pressure). The sample holder is sealed and the sample heated to the aging temperature T2. Once the temperature is stabilized, then sample is allowed to age for a certain period of time (Right). If there is disproportionation, then the pressure rises and H/M decreases as function of time.
In general aging results are comparable with long-term cycling results. It is worth noting however, that isotherm results after aging tests are similar but not exactly the same as in long-term cycling tests for intermetallics, in particular for LaNi5, an example of this is shown in the following section. An important exception to the ability of aging tests to simulate the effects of cycling is in cases where there is compositional inhomogeneity of the starting alloys or the alloy was not properly annealed. Aging will produce different results as will later be demonstrated in the case of LaNi5 micro-alloyed with Al.
In general, it is assumed that degradation due to disproportionation (the decomposition of the alloy into more stable hydrides or alloys) results from cycling alone. However, it is possible to achieve these results by simply subjecting the hydrides to static higher hydrogen pressures than the isotherm plateau pressure for a particular temperature. This process is referred to as “Thermal Aging”. Sandrock et al. showed the disproportionation of LaNi5H6 could be reversed through Reproportionation Aging (RPA) by holding the sample under vacuum for a certain period of time, and then cooling it down to room temperature. Isotherms of disproportionated and reproportionated hydrides show dramatic changes. Changes in the slopes and H/M capacities allow some assessment of property changes under different conditions. They suggested that significant disproportionation occurs only if the pressure is significantly above that of the plateau pressure. In Figure 5, isotherms of LaNi5 were taken after aging at 136, 61, 10, atm, and under vacuum at 180 oC. It can be seen that high-pressure aging leads to sloping plateaus but when vacuum aged (annealed), one observed nearly flat plateau and properties are more or less recovered. It should also be noted that there are some reports of the restoration of hydriding properties by a different process involving cycling.

Figure 5: Isotherms of LaNi5 taken at 25°C after subjecting to aging at high and low hydrogen pressures. Note that the isotherm for vacuum aged sample at 180°C is nearly (but not exactly) the same as the isotherm before aging indicating full reproportionation of the LaNi5 hydride with a flat plateau after vacuum aging.
In the past, cycling and aging tests were not optimized based on first using this aging/isotherm measurement approach. Pressures and temperatures were simply based on extreme conditions rather selecting the best settings based on such data to accelerate the aging process.
6. Intrinsic Testing of Intermetallic Hydrides (Thermal, Pressure and P-T Cycling Tests)
Intrinsic cycle testing of hydrides involves using the same gaseous hydrogen over and over again with every absorption/desorption cycle. Pure hydrogen is typically used, but it can be extended to industrial or laboratory hydrogen gas as long as the fresh gas is not added at every cycle. These types of degradation studies are carried out for alloys or intermetallic hydrides that are used for heat pumps and other applications, where the same hydrogen is recycled back and forth. Using this degradation method, one can examine the long-term intrinsic behavior of elemental, binary, ternary hydrides in the absence of any gas impurity effects.
For these tests to be valid, it is clear that the apparatus used must be completely free of leaks because the introduction of even ppm levels of impurities (air) nullifies the assumption of purely intrinsic properties. This means that all components must be able to withstand the pressure and temperature conditions of the cycle tests without leaking (i.e. through thermal expansion of seals) or out-gassing at elevated temperatures. For this reason metal-on-metal seals and hydrogen compatible metal components (i.e., constructed from 316L stainless steel) are highly recommended.
Reference List:
Sandrock, G., “A panoramic overview of hydrogen storage alloys from a gas reaction point of view”, J. Alloys and Compounds, 293-295
Lambert, S.W., Chandra, D., Cathey, W.N., Lynch, F., E., and Bowman, Jr., R., C., “Investigation of hydriding properties of LaNi4.8Sn0.2, LaNi4.27Sn0.24 and La0.9Gd0.1Ni5 after thermal cycling and aging”, J. Alloys and Compounds
Bowman, Jr., R.C., “Development of Metal Hydride Beds for Sorption Cryocoolers in Space Applications”, J. Alloys Compounds
Aoki, K., Yanagitani, A., and Masumoto., T., “Crystalline to Amorphous Transformation in Laves Phase GdFe2 Induced by Hydrogen Absorption”, Applied Physics Letters
Ahn, S.T., Kim, Y.G., and Lee., J.Y., “Formation of the Amorphous Phase in Zr2Al by Hydrogen Absorption and the Effects of Titanium Substitution on the Amorphization Behavior”, J. Alloys and Compounds
Aoki, K., Yamamoto, T., and Masumoto, T., “Hydrogen Induced Amorphization in RNi2 Laves Phases”, Scripta Metallurgica
Kim, Y.G. and Lee., J.Y., “Hydrogen-Induced Transformation to an Amorphous State in the Laves Phases Ce(Ru, M)2 (M Equivalent- to Fe, Co, Ni)”, J. Alloys and Compounds
Sandrock, G.D., Goodell, P.D., Huston, E.L., and Golben, P.M., “On the Disproportionation of Intermetallic Hydrides”, Z. Für Phys. Chem. Neue Folge
Cohen, R.L. and West K.W., “Intrinsic Cycling Degradation in LaNi5 and Annealing Procedures for Re-forming the Material”, J. Less-Common Metals
All Rights Reserved by Gold APP Instruments Corp. Ltd.
WeChat WhatsApp


GOLD APP INSTRUMENTS CORP. LTD.
HongKong Add: Flat Rm A17, Legend Tower, No. 7 Shing Yip Street, HK, China
Mainland Add: R1302, Baoli Tianyue, Shaowen Rd., Yanta Dist., Xi'an 710077, China
T: +86-182 0108 5158
E: sales@goldapp.com.cn